Projects
At BSIM Therapeutics, we are pairing cheminformatics, molecular modeling, data mining and machine learning techniques with in vitro and in vivo validation to discover potent and selective transthyretin stabilizers, holding physicochemical properties suitable for acting in multiple target organs – including the eye, the brain and the blood plasma.
AMILOTERA



AMILOTERA CENTRO-07-0202-FEDER-021622
Approved on July 11, 2011 for the reinforcement of research, technological development and innovation. Project supported through the National Strategic Reference Framework (QREN).
Start Date:
02/01/2012
End Date:
30/06/2015
Total Eligible Value:
707.006,84 €
EU Funding:
FEDER 500.000,00 €
Development and optimization of therapeutic agents against transthyretin amyloidoses
Beneficiary: consortium BSIM Therapeutics (leader) / UC / CNC
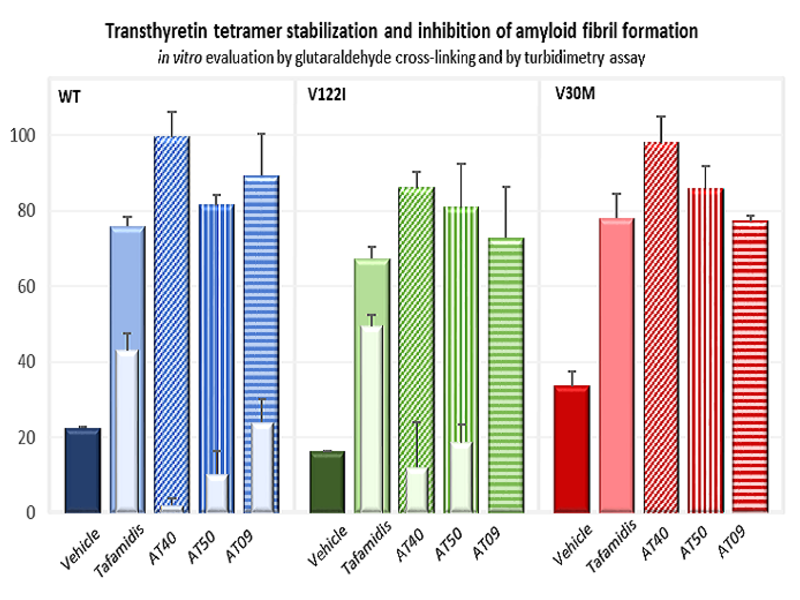
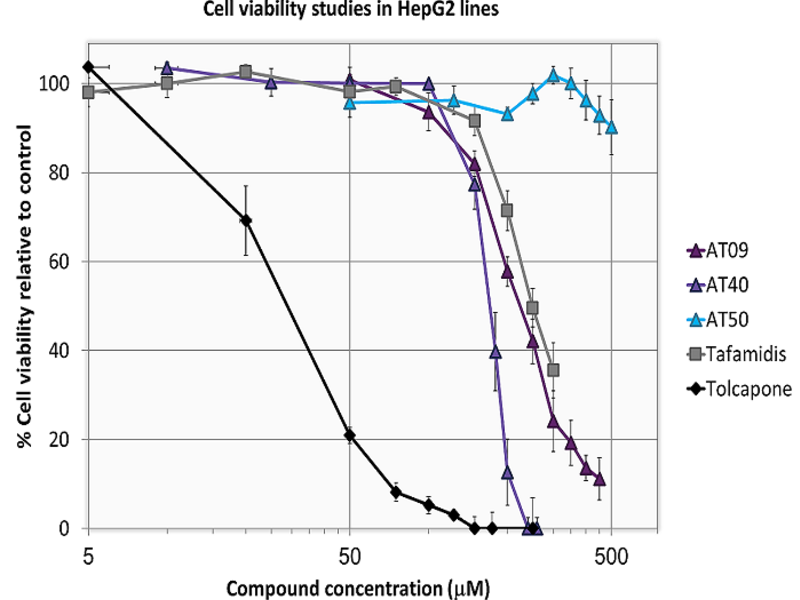
Discovery of novel inhibitors of transthyretin amyloid fibril formation. More than 100 mutations in the transthyretin (TTR) gene are linked to amyloidosis, with the V30M and V122I variants being the most common in hATTR-PN and hATTR-CM, respectively. Mutations affect not only the stability and deposition sites of the TTR protein, but also TTR’s propensity to associate with small organic molecules. Most molecules currently under test, including the EMA-approved drug, display modest levels of stabilization of the most unstable TTR variants. The AMILOTERA programmed paved the way to the discovery of three lead compound series with unprecedent pharmacological potential, displaying inhibitory activity against multiple variants of TTR.
NEUROTERA



NEUROTERA POCI-01-0247-FEDER-003431
Date of approval: 22/02/2016. Main objective: To reinforce Research, Technological Development and Innovation. Intervention Region: North, Center. Project supported by COMPETE 2020 of PT2020, through the European Regional Development Fund.
Start Date:
16/08/2016
End Date:
15/02/2020
Total Eligible Value:
1.136.307,60 €
EU Funding:
FEDER 891.220,51 €
Development of innovative drugs against ocular and cerebral co-morbidities associated to Familial Amyloid Polyneuropathy
Beneficiary: consortium BSIM Therapeutics (leader) / UC / IBMC
Targeting potent TTR amyloid inhibitors to meet multiple clinical manifestations. Loss of visual acuity, ischemic events and cerebral bleeding are today part of the clinical spectrum of TTR-FAP and its treatment is not addressed by currently known/available therapies. The NEUROTERA programme aims at exploiting a series of potent TTR amyloid inhibitors discovered throughout our AMILOTERA programme, by targeting selected drug candidates to the EYE and the BRAIN, for the treatment of the clinical manifestations linked with those organs.
TERAPI-1



TERAPI-1 CENTRO-01-0247-FEDER-028007
Approved on October 20, 2017 to strengthen research, technological development and innovation. Project supported by CENTRO 2020 of PT2020, through the European Regional Development Fund.
Start Date:
20/05/2017
End Date:
19/05/2021
Total Eligible Value:
48.768,66 €
EU Funding:
FEDER 24.384,33 €
Intellectual property protection of new therapeutic agents (AT50) against transthyretin amyloidoses.
Beneficiary: BSIM Therapeutics
Protecting our knowledge. Securing protection of intellectual property rights in several territories is fundamental to our company’s business strategy, allowing commercial licensing of our products and future growth. Having attained proof-of-principle against several disease-modifying endpoints with our best TTR stabilizers, each with licensing potential, we have shown that it is possible to develop innovative next-generation small molecule therapies against transthyretin (TTR) amyloidoses. The project CENTRO-01-0247-FEDER-028007 aim at supporting the patenting of the AT50 technology.
TERAPI-2



TERAPI-2 CENTRO-01-0247-FEDER-032687
Approved on October 20, 2017 to strengthen research, technological development and innovation. Project supported by CENTRO 2020 of PT2020, through the European Regional Development Fund.
Start Date:
01/06/2017
End Date:
31/05/2020
Total Eligible Value:
50.000,00 €
EU Funding:
FEDER 25.000,00 €
Intellectual property protection of new therapeutic agents (AT40) against transthyretin amyloidoses
Beneficiary: BSIM Therapeutics
Protecting our knowledge. Securing protection of intellectual property rights in several territories is fundamental to our company’s business strategy, allowing commercial licensing of our products and future growth. Having attained proof-of-principle against several disease-modifying endpoints with our best TTR stabilizers, each with licensing potential, we have shown that it is possible to develop innovative next-generation small molecule therapies against transthyretin (TTR) amyloidoses. The project CENTRO-01-0247-FEDER-032697 aim at supporting the patenting of the AT40 technology.
TERAPI-3



TERAPI-3 CENTRO-01-0247-FEDER-037867
Approved on June 06, 2018 to strengthen research, technological development and innovation. Project supported by CENTRO 2020 of PT2020, through the European Regional Development Fund.
Start Date:
15/12/2017
End Date:
14/12/2021
Total Eligible Value:
50.000 €
EU Funding:
FEDER 25.000 €
Intellectual property protection of new therapeutic agents (AT09) against transthyretin amyloidoses.
Beneficiary: BSIM Therapeutics
Protecting our knowledge. Securing protection of intellectual property rights in several territories is fundamental to our company’s business strategy, allowing commercial licensing of our products and future growth. Having attained proof-of-principle against several disease-modifying endpoints with our best TTR stabilizers, each with licensing potential, we have shown that it is possible to develop innovative next-generation small molecule therapies against transthyretin (TTR) amyloidoses. The project CENTRO-01-0247-FEDER-037867 aim at supporting the patenting of the AT09 technology.
TERAPI-4



TERAPI-4 CENTRO-01-0247-FEDER-046897
Approved on April 16, 2020 to strengthen research, technological development and innovation. Project supported by CENTRO 2020 of PT2020, through the European Regional Development Fund.
Start Date:
01/04/2020
End Date:
01/04/2023
Total Eligible Value:
50.000,00 €
EU Funding:
FEDER 25.000,00 €
Intellectual Property protection of new therapeutic agents against transthyretin amyloidoses: NT60 technology.
Beneficiary: BSIM Therapeutics
Protecting our knowledge. Securing protection of intellectual property rights in several territories is fundamental to our company’s business strategy, allowing commercial licensing of our products and future growth. Having attained proof-of-principle against several disease-modifying endpoints with our best TTR stabilizers, each with licensing potential, we have shown that it is possible to develop innovative next-generation small molecule therapies against transthyretin (TTR) amyloidoses. The project CENTRO-01-0247-FEDER-046897 aim at supporting the patenting of the NT60 technology.